Folding cartons – indispensable for medicinal products

Article published in pharmind on January 2015
Authors: Prof. Dr. Martin Angerhöfer und Dipl. Ing. Roland Kleissendorf
The demands on folding cartons for medicinal products have increased steadily in the past, and will continue to do so in the future. If these demands are to be met in everyday practice, it will become increasingly important to understand the material-specific characteristics of folding cartons and how they can be affected by changes at every stage, from production through to their use by the final consumer. This article provides an overview of the key requirements for folding cartons in terms of their efficiency in the packaging process, suggests solutions to problems that may occur, identifies potential areas for optimization and concludes by outlining the demands that will need to be met in the future when specifying folding cartons for medicinal products.
Increasing demands
The main function of folding cartons as secondary packaging for medicinal products is to provide protection for the packaged products and to store information about them. Folding cartons must also enable the packaging process to be carried out cost-effectively and without problems. In the recent past further requirements have been added. These include the application of braille, design regulations like the “blue box” for central registration through the European Medicines Agency (EMA), special colours for hazard warnings, additional adhesive labelling and an increasing call for variable data to be printed on the boxes as they pass through the packaging machine. In addition, constantly increasing demands are being placed on packaging performance, while at the same time orders are becoming smaller and order cycles generally shorter. Overall there is an increasing trend for folding cartons to be used to meet logistical needs throughout the entire supply chain. The demands made on folding cartons for medicinal products have been increased considerably by EU Directive 2011/62/EU (the “Falsified Medicines Directive”) and similar legislation in other countries. Barcodes and 2D matrix codes now have to be applied by the folding carton suppliers or in the packaging machine. This information then has to be read accurately in the packaging plant and the packs grouped into logistical units (bundles or shipping units). This enables seamless monitoring of a medicinal product all the way from the packaging company to the customer/patient. Under the EU Falsified Medicines Directive, folding cartons are also required to have a tamper-evident closure. With the current state of the art this can be achieved by incorporating additional flaps when gluing the folding cartons together, using adhesive labels or adopting other design features.
Optimizing folding cartons by selecting the right type of board
Paperboard is the basic raw material for folding cartons. It is available on the market in a variety of different qualities and basis weights. A list of grades is published by the FFI (Fachverband Faltschachtel-Industrie – German Folding Carton Industry Association (www.ffi.de)). The most important types of paperboard for packaging medicinal products are GC (primary fibre- or virgin fibre-based board) and GD or GT (secondary-fibre board, i.e. recycled material). The usual basis weights range from 220 to 380 g/m².
This brings us to one of the most important aspects of material selection. Although the specifications for paperboard always indicate its basis weight, this does not constitute a technical characteristic. The most important indicator of the stability of a pack is the bending stiffness of the paperboard. This is the resistance that a material offers to a bending load, and essentially determines its load-bearing capacity and technical performance during the packaging process. A distinction has to be made between bending stiffness in machine direction (MD) – i.e. the production direction of board in the paperboard machine – and bending stiffness in cross direction (CD). Depending on the type of board machine, the MD/CD ratio can vary between 2.5 and 1.8. Experience to date shows that a high CD bending stiffness combined with a low MD/CD ratio is advantageous for the packaging process.
When selecting the type of board – and thus when specifying the packaging material – bending stiffness must be the prime consideration. The basis weight can be ignored, or at best provided as additional information. In everyday practice this means that if problems can be attributed to inadequate stability of the folding carton, the bending stiffness of the board must be tested and if necessary increased. An increase in bending stiffness does not necessarily involve an increase in the basis weight (and thus the cost) of the board. In some cases it is possible to alter the quality of the board and achieve a higher bending stiffness without altering the basis weight. A look at the bending stiffness quickly reveals where there is potential for reducing the amount of material used and hence the cost. Our own experience in packaging production and the observations we have made during our consultancy projects show that many folding cartons for medicinal products are over-specified with respect to the quality of board used.
Reducing the amount of packaging material is a very complex task. The aim is to use as little packaging material as possible, while at the same time ensuring that the packaging – and of course the packaged product – remains undamaged, and preventing any disruption to the packaging machines. In this context it is important to note that using less than the optimum amount of material can lead to a far greater increase in costs than would be the case if the optimum amount of material were exceeded by the same amount (Fig. 1). This is because under-specifying leads to an exponential increase in costs as a result of damage to the product or reduced packaging efficiency, whereas over-specifying merely leads to a linear increase in costs (for paperboard, transport and waste disposal).
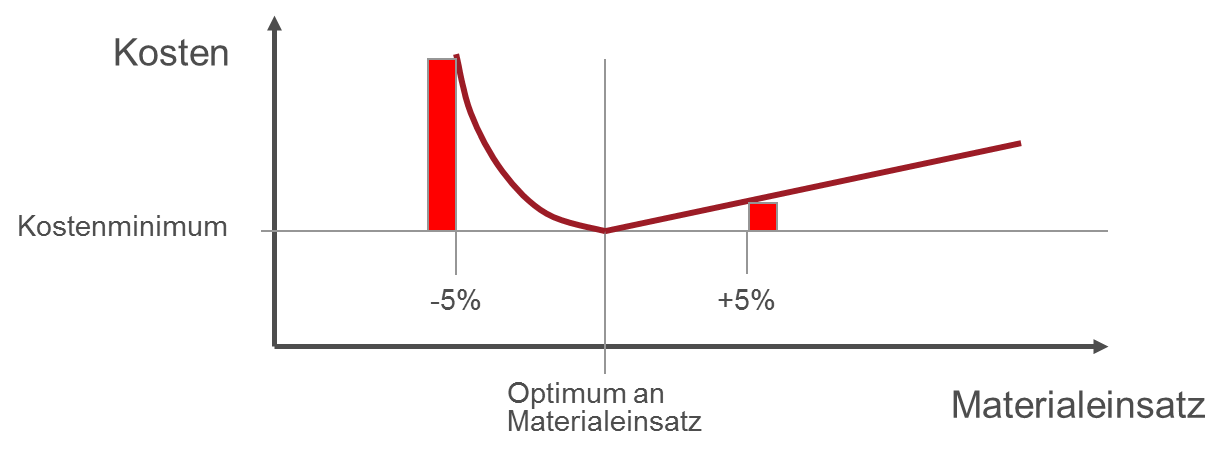
Fig. 1: Packaging costs as a function of material use
As mentioned earlier, the key parameter when specifying folding cartons for medicinal products is the cross-directional bending stiffness. Figs 2 and 3 show how this fact can be used to ensure selection of the optimum type of paperboard. Fig. 2 plots the cross-directional bending stiffness as a function of basis weight for various GC paperboard grades widely available on the European market. It is immediately obvious that one and the same basis weight can offer a considerable range of bending stiffness.
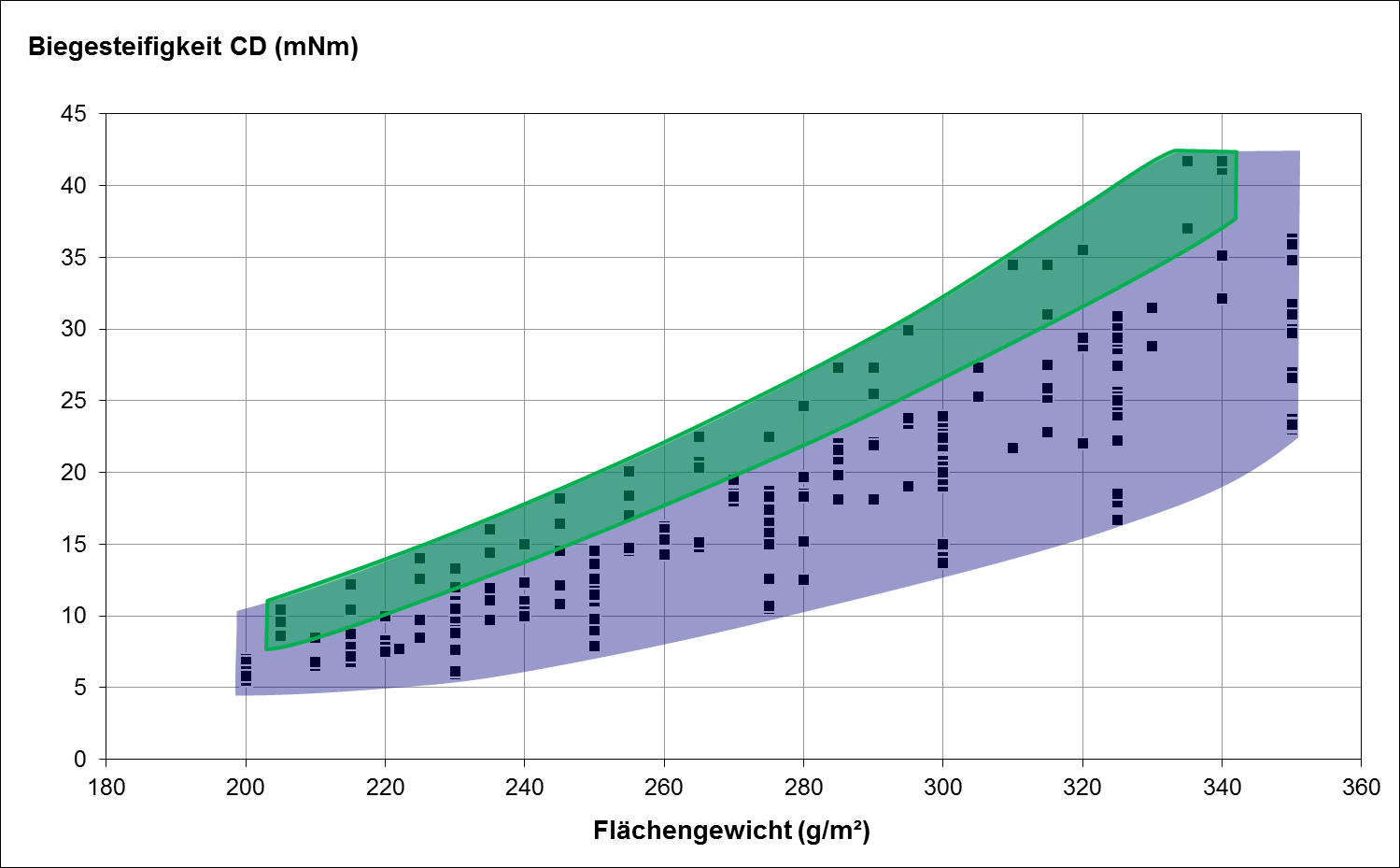
Fig. 2: Cross-directional bending stiffness of different paperboard grades
If we assume that a minimum board bending stiffness of, for example, 15 mNm (milli-Newton x meter) is required for a given folding carton to go through the packaging process without causing any problems (the minimum value depends primarily on the dimensions of the folding carton), and if we list the basis weights of different board grades that meet this minimum requirement, we obtain Fig. 3. If the grade of paperboard selected is unsuitable for use as a packaging material, a basis weight of 325 g/m² is needed; if an optimum grade of paperboard is chosen, however, a basis weight of 235 g/m² is sufficient – a weight saving of more than 25 %.
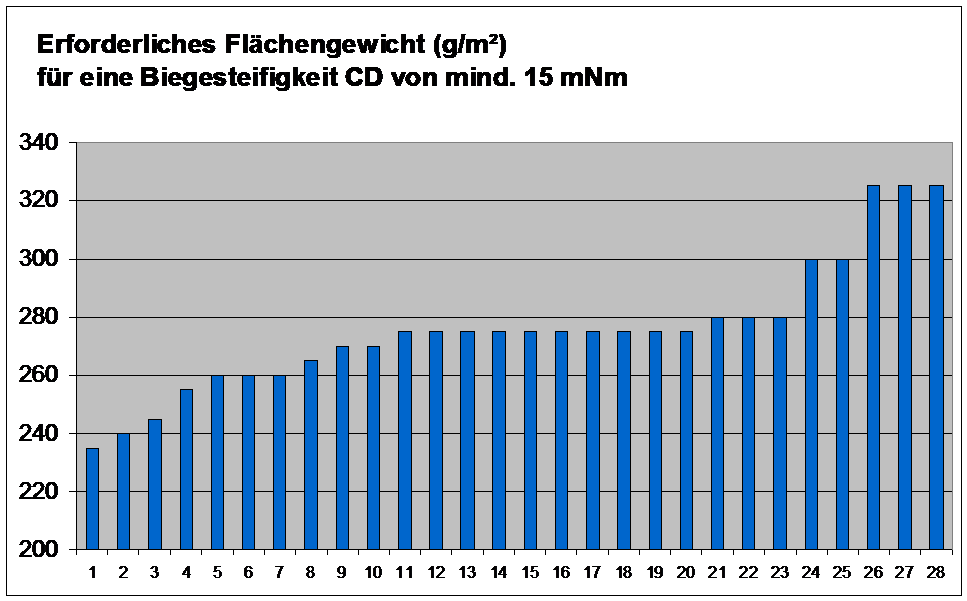
Fig. 3: Basis weights necessary for a minimum CD bending stiffness of 15 mNm
In practice the bending stiffness can be classified in terms of minimum CD values (CD min.) and maximum thickness values (D max.). Depending on the specified limit values, a varying number of paperboard grades meet these requirements (see green shaded area of Fig. 2). Experience shows that it makes sense to limit the choice to a few paperboard grades with a similar bending stiffness / thickness ratio.
Over the years empirical approaches and trial-and-error methods have been used in an attempt to find the ideal material. These attempts have naturally been more intensive in areas where the packaging costs account for a high proportion of the total product costs, for example, in the food industry. For many years now the tobacco industry has been working on ways of reducing the weight of packaging. Its success can be measured by the fact that 20 years ago the average basis weight of a cigarette pack made of paperboard was 230 to 240 g/m², whereas today basis weights of 200 to 210 g/m² are normal, and packaging machine output has tripled. In the pharmaceuticals industry there are many cases where potential savings of up to 25 % on the grade of paperboard used and thus of up to 10 % on the cost of the folding cartons could be achieved.
The importance of board moisture
Unlike most packaging films, cardboard is hygroscopic and therefore interacts to an appreciable degree with ambient humidity. Absorbing or releasing moisture causes changes in the physical properties of cardboard, such as bending stiffness and its folding behaviour at the creases. Fluctuating climatic conditions are particularly critical. They cause irreversible structural changes which can lead to serious packaging problems, depending on the degree of variation in the humidity. It is therefore essential to keep folding cartons at a constant level of atmospheric humidity, both when in storage and during the packaging process. In our experience the limit values for relative humidity are between 40 and 60 %. These are also the conditions required for the production of folding cartons, and which are maintained, for the reasons given above, through air-conditioning of the production and storage areas.
Long storage times can also have an adverse effect on packaging performance. Folding cartons are generally supplied flat and pre-coated with adhesive, with two of the longitudinal creases being pre-folded through 180 °. The longer they are kept in storage, the more these creases lose their resilience, which is crucial if the boxes are to return to an upright position in the carton erector. Resilience also decreases over time if the folding cartons are packed too tightly in the shipping boxes or if the folding pressure in the gluing machine of the folding box manufacturer is too high. The force needed to erect the flat folding cartons at the crease lines (which must be pre-folded at the gluing stage) must not be too high, and here too lengthy storage times can have a detrimental effect. All these factors are also negatively influenced by unfavourable climatic conditions.
In our experience, the primary causes of poor packaging performance are exposure to moisture during storage and/or production and long storage times. Obvious remedies for this are humidity controls and shorter order cycles. The need here is for good procurement logistics which are adapted to the logistics of the packaging plant and require the folding carton supplier to provide boxes of the same type (in terms of design, printing, etc.) on a rolling basis. In many cases manufacturers of medicinal products leave it to their suppliers to produce follow-up batches of the same type of folding cartons. Any order optimisation practised there, however, is generally geared only to the production process of the folding carton manufacturer. The “just in time” delivery of precise numbers of units, as is common in other industries, is not generally found in the pharmaceuticals sector. In the case of repeat orders this gives rise to a situation where fresh folding cartons are processed together with those that have been in storage for a long time.
Other factors affecting packaging machine efficiency
In addition to key parameters like bending stiffness and humidity, a number of other influences must be taken into account when processing folding cartons.
Beispielsweise ist bei großer Staubbelastung in der Verpackungsmaschine, insbesondere bei hohen Losgrößen zu prüfen, ob die verwendete Stanze mit neuen Schneidlinien zu versehen ist, um glatte, saubere Schnitte zu erhalten. Ursache von übermäßigem Staub kann aber auch die Zusammensetzung des eingesetzten Kartons sein.
For example, the presence of large amounts of dust in the packaging machine, particularly when large batches are being manufactured, may indicate that the die-cutting machine needs new cutting rules in order to ensure smooth, clean cuts. Excessive dust can, however, also be caused by the composition of the paperboard used.
The subsequent labelling of folding cartons with 2D matrix codes and a description of their contents in legible lettering requires completely new specifications for the surface of the paperboard. Any solvent- or water-based inks can be used for printing, but in all cases rapid ink drying or solvent absorption is essential. UV-curing inks are another option. Ink-removing lasers are also used. All these methods have advantages and disadvantages and must be suited to the paperboard material. Ground-breaking work in this area has been carried out by the FFPI (Forschungsgemeinschaft Faltschachteln für die Pharmazeutische Industrie – Research Association for Folding Cartons for the Pharmaceuticals Industry) in collaboration with the Papiertechnische Stiftung (Paper Technology Foundation) in Munich.
Occasional problems with the transportation of the folding cartons through the packaging machine may be caused by unsatisfactory frictional behaviour. The determining factor here is the formulation of the lacquer (usually water-based, less often UV-curing). Interactions with the paperboard and any primer coatings used must also be taken into account. Excessive “powdering” during printing can also have a detrimental on the surface of the folding carton. At present, however, no reliable methods for specifying and testing the relevant coefficients of friction are available.
For trouble-free erection of the folding cartons in the packaging machine there should be at least 3 mm difference between the A and B dimensions. This is to ensure that the longitudinal creases do not lie on top of one another and the folding cartons do not fold in the wrong direction when erected. In the case of folding cartons with a significantly larger B dimension, stabilising measures such as additional creases on the lid flaps can help prevent such problems. Our investigations have shown that dimensional variations in folding cartons are of secondary importance. The precision of modern, laser-cut tools for folding carton manufacture generally rules this out as a source of error.
Of the factors we have mentioned above, which ones are actually responsible for the occurrence of problems during packaging must always be determined by accurate analysis of the type and location of the fault. With the high packaging speeds that are customary today, video footage taken using high-speed cameras and then slowed for viewing can be very useful when attempting to pinpoint sources of error.
Standardising dimensions to reduce costs
The dimensions of folding cartons used in the pharmaceuticals sector are generally tailored to the standards of the primary packaging. As these standards are mainly company-specific, from an overall industry point of view standardization is less than perfect. Attempts to improve this situation have met with limited success. In this connection we would like to make particular mention of our work in the FFPI, which has put forward a proposal worthy of consideration in the form of PAS 1009 (Publicly Available Specification – published by Beuth).
As far as folding carton construction is concerned, standardisation was better disseminated and implemented. DIN Standard 55429 Part 1 contained specifications for the construction, dimensions (A x B x H), limit deviations and testing of folding cartons. Customised designs could be specified on the basis of this standard. Unfortunately it was replaced in 2003 by a standard (DIN EN 14054) that was less suitable for folding cartons intended for medicinal products. The only solution now is to incorporate the content of DIN 55429 Part 1 into individual company specifications. It also makes sense to use the CAD data compiled by the folding carton manufacturer on the basis of these specifications as a template for typesetting.
There will be opportunities to take over the contents of the former DIN Standard, or even to introduce a new form of standardisation, when the folding carton standards are reframed following the introduction of the EU Falsified Medicines Directive and whenever the dimensions of folding cartons are altered by the demands of printing (EMA standard texts) and by the increase in size of the package inserts. Projects focused on future needs in this area need to be started now!
Summary
As this paper shows, folding cartons are becoming increasingly important for packaging medicinal products. The potential for optimisation in terms of specifications and costs is far from exhausted. Whether it is a matter of optimisation in particular areas or finding rapid solutions to problems, the challenges can only be met by focusing attention on the areas we have highlighted.
With a wealth of practical experience acquired over many years, the authors will be glad to assist any interested companies in this field on a consultancy basis.

angerhoefer consulting
Prof. Dr.-Ing. Martin Angerhöfer
Eichenweg 17, 88289 Waldburg


